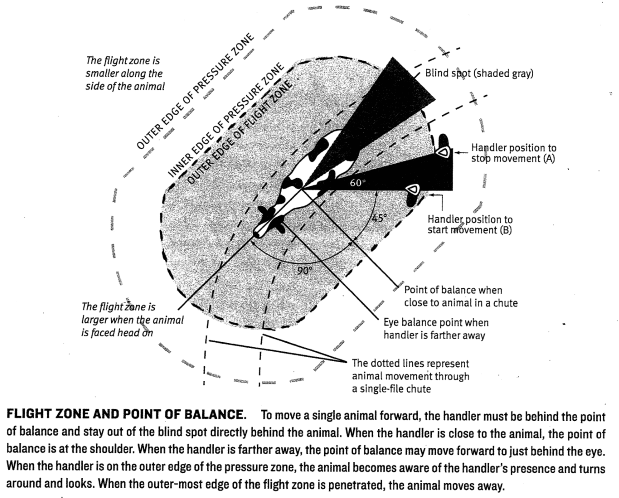
Figure 4.1: Flight zone diagram showing the correct positions for the handler to move an animal.
Writing on the behavioral aspects of animal domestication for the Quarterly Review of Biology Price (1984) stated:
It is difficult to generalize about the effects of domestication on either genetic or phenotypic variability because of different selection pressures on different traits and species. However, it is apparent that, with respect to animal behavior, domestication has influenced the quantitative rather than the qualitative nature of the response.
To put it in simpler words, domestication changes the intensity of a behavioral response. For example, zebras are more flighty and nervous than domestic horses. Parsons (1988) further wrote that domestic animals are more stress resistant because they have been selected for a calm attitude toward man. In either event, a genetic predisposition to be fearful or calm interacts with early experience and learning in very complex ways. The term temperament will be used in this chapter to refer to nervous system reactivity, which is determined by both genetic and environmental factors. During handling, fear is a major determinant of an animal's behavior. Genetic influences on temperament interact with early experience and learning to shape adult patterns of behavior. The most convincing examples of genetic influence on behavior come from selection and strain studies of behavior in mice (DeFries et al., 1978). Selective breeding of rats for either high or low fearfulness clearly shows that genetics plays a major role in determining an animal's temperament (Broadhurst, 1960; Eysenck and Broadhurst, 1964; Huck and Price, 1975). Behaviors studied in the laboratory are clearly shown to be substantially influenced by genetic factors (Plomin, 1990). In the following sections we will discuss the interacting forces of genetics and experience. Some of the topics which will be covered are flocking behavior, herding behavior, flight zone, social behavior, attraction and reaction to novelty, inheritability of behavior, and sire effect; some general principles for handling domestic animals will also be discussed.
The first author has observed that wild ungulates such as the American bison have stronger herding behavior compared to domestic cattle. Separation of a single animal from the herd will cause it to make an intense effort to rejoin its herdmates (Grandin, 1993). Domestic cattle become more difficult to handle and separate when they become agitated and engage in "shelter-seeking behavior." Each animal will attempt to push itself into the middle of the group where it will be safe from predators. The strongest animals will end up in the middle of the milling herd. In their natural environments, prey species animals such as elk and deer will spread apart when grazing a hillside, but at the first sign of danger they will group closer together and flee as a herd. Domestic herding animals display similar behavior. When herding animals sense danger they tend to flock together. The smaller hoofed animals tend to be more reactive and will herd together more tightly than the larger ones. For example, sheep are more vulnerable to predators than cattle, and therefore bunch together very tightly to find safety in numbers.

Figure 4.1: Flight zone diagram showing the correct positions for the handler to move an animal.
Genetic factors influence individual flight zone sizes between animals of the same species or breed. For example, in a large group of animals with similar genetics and previous handling experiences, most members of the group will have similar flight distances, but a few individuals will have either a very small or a very large flight zone. Flight zone size is also strongly affected by experience and learning. An animal that survives a close call with danger learns to be more wary of similar dangers in the future and increases its flight zone size accordingly. Furthermore, a completely tame or trained animal has no flight zone and will allow people to approach and touch it (Price, 1984). Cattle that have people moving around them everyday will have smaller flight zones than genetically similar cattle raised on ranches in the mountains who seldom see people. Thus, interactions between genetics and experience in shaping an animals flight zone size can be complex.

Figure 4.2 Collective flight zone of a large flock of sheep. As the people walk through, the sheep move away and circle around them.
Dogs trained for herding use the principles of the flight zone and the same positions described above to control livestock movement. Coppinger and Coppinger (1993) propose that dogs which herd livestock in this manner are displaying a natural hunting strategy used by wolves. Fortunately for the livestock, though, domestic dogs have been bred so that they do not attack or kill.
These principles are familiar to most people that handle or raise domestic cattle and horses. Horse trainers use trained horses that are calm to help ease the fear in young horses of novel experiences. For example, loading in a trailer for the first time, being ridden for the first time, or even the stress of weaning can be reduced by having a calm companion horse nearby. In Australia, tame "coacher" cattle are used to assist in gathering wild feral cattle (Roche, 1988). Fordyce (1987) also used a few tame steers to facilitate training Brahman calves to novel handling procedures. On "Old West" cattle drives, lead steers with a calm temperament were kept around and excitable leaders were destroyed (Harger, 1928). Excitable leaders caused stampedes.
Cattle with Brahman genetics are generally considered flighty compared to breeds with British genetics such as Angus and Hereford. However, Brahman cattle are the most inquisitive and will investigate or follow a person or a dog. A common practice used in Australia to move groups of Brahmans is allowing them to follow a person. The tendency to follow a person is greater in Brahman compared to British or European Continental breeds. Nevertheless, Hohenboken (1987) found that if Brahmans are handled gently they can become extremely docile. Murphey et al. (1980, 1981) also discussed behavioral differences between several breeds in their flight zone size, and in their tendency to approach novel objects or a man lying on the ground. The breeds with the largest flight zones had the strongest tendency to approach novel objects. This is only true when the animals voluntarily approach the novel object. During forced movements where the animals are being driven toward a novel object just the opposite is true. The excitable flighty individuals will be most fearful and they will be more likely to "spook" or balk.
In 1993, the first author began to receive reports from cattle feeders in the United States about certain cattle that were unable to adapt to a feedlot. Newly arrived cattle in a feedlot usually adapt to vehicles and people within a few days. However, feedlot operators began to notice that some crossbreeds with European Continental genetics were never able to adapt to the feedlot. Some of these animals had to be removed from the feedlot and returned to pasture. Further observations by the first author suggest that the behavior of highly reactive excitable European Continental crosses during handling or restraint is different from the behavior of Brahman or Brahman cross cattle. For example, when Brahman cattle become agitated they are seldom self-destructive. However, the first author has observed that European Continental crosses are more likely to injure themselves if they panic during handling in squeeze chutes or truck loading ramps. Brahman cattle are more likely to lie down and become immobile. Certain genetic lines of European Continental cattle are more excitable than British breeds (Grandin, 1994; Stricklin et al., 1980). The second author has made similar observations in both calm and high-strung breeds of horses. The more reactive high-strung breeds such as Arabians or Thoroughbreds are more likely to panic compared to the more placid and less fearful breeds such as draft horses.
The time has come to stress the existence of genetic differences in behavior, in view of the enormous amount of material the students of various forms of learning have accumulated on non-genetic variation in behavior. Striking individual differences have been described for predator-prey relationships, for the reactions of birds to mimicking or to warning colorations, for childcare among primates, and for maternal behavior in rats. It is generally agreed by observers that much of this individual difference is not affected by experience but remains essentially constant throughout the entire lifetime of the individual.
Individual differences in animal behavior have been widely discussed by Hirsh (1963), Scott and Fuller (1965), Fuller and Thompson (1978), and Plomin (1990). Livestock animals show individual differences in behaviors similar to those found in laboratory animals and dogs. Kerr and Wood-Gush (1985) investigated a relationship between individual behavioral differences and production measures in dairy cows. They propose that the behavior of dairy heifers in one context can be used to predict behavior in other situations. Furthermore, Kerr and Wood-Gush (1987) found that an individual dairy heifer's reaction to being touched by a human observer showed a consistent individual pattern, which remained consistent for a 2-year period.
In his experience training horses, the second author has observed similar individual differences in the temperament of horses. High-strung fearful horses often take several years to habituate and stop fearing a novel situation such as a new riding arena, transport vehicle, or a strange person. Calmer, more placid animals will habituate more easily to a novel stimulus than high-strung, flighty animals.
An open field test or flight zone test can also be used to assess temperament. Kilgore (1975) used the open field test as an assessment of temperament in dairy cows; Le Neindre et al. (1995) documented individual differences in the docility of Limousin cattle. In this study individual cattle were separated from their herdmates by a person working their flight zone. Individuals were considered aggressive if they lowered their heads or charged, the handler. A passive flight zone test can also be used. In this test a person stands or sits quietly and waits for animals to voluntarily approach. Animals with a calm temperament and low fear of humans will approach more closely and fearful animals will have a larger flight zone. Murphey et al. (1980) found that he could approach the dairy breeds more closely than the beef breeds.
Grandin (1992) assessed the temperament of bulls, which were restrained in a squeeze chute four times at 30-day intervals. She concluded from this study that to obtain accurate temperament evaluations on individual animals they should be scored more than once. Temperament scores were stable over time for the calmest and the most excitable animals. Temperament scores taken in a squeeze chute may more accurately show the animals true physiological reactivity compared to passive flight zone tests.
The first author has observed that confronting an animal with a sudden novel or aversive experience is more likely to make it show its true reactivity Flight zone is possibly more affected by learning than reaction to a sudden aversive event. For example, Herefords, which are a calm, placid breed, may have large flight zones if they are raised where they seldom see people, but even a very wild, extensively raised Hereford will seldom become highly agitated and struggle violently in a squeeze chute. On the other hand, a tame halter broke individual of the U.S. genetic lines of Saler or Limousin is much more likely to panic when suddenly confronted with novelty compared to an extensively raised Hereford.
The handling tests used by Lawrence et al. (1991) were: (1) willingness to leave the home pens, (2) movement ease through a hallway, (3) response to a suddenly approaching human, (4) restraint-resistance using a snout snare, and (5) vocalization during restraint. With the exception of vocalization, all the tests had a strong tendency to correlate. The single best predictor of temperament in the pigs was the suddenly approaching human who stamped his foot. The response was scored on a five-point scale: (1) no response, (2) mild flinch, (3) animal backed away from the person, (4) animal backed away and squealed, and (5) animal attempted to escape. Lawrence et al. (1991) concluded that the temperament differences between individuals were stable over time.
The first author has observed that very lean hybrid pigs are much more excitable during handling than fatter breeds of pigs. Ultra-lean hybrid pigs with very little back fat became popular in the United States during the mid 1990s. From her experience, she speculates that this effect cannot be attributed entirely to early rearing environments. Striking differences in pig behavior occur in lean hybrids, which were raised in the same building operated by the same people. Many pork producers have commented that lean hybrids are more excitable than older crossbred pigs with large amounts of back fat. The differences in behavior were first noticed when producers changed breeding stocks. Lean hybrids are more reactive and more likely to squeal when touched. Pigs with a very excitable temperament are much more difficult to handle (Grandin, 1994). The first author has observed that they can be moved easily if the handler moves slowly and avoids sudden movements. Sudden movements cause excitable pigs to bunch together and pile up.
At the slaughter plant lean hybrids cause serious handling, problems in plants which process 1000 pigs per hour on a single line. The highly reactive pigs balk more, and are more difficult to drive. Several large slaughter plants have had to install a second complete handling and stunning system in order to handle the lean hybrids at a rate of 500 pigs per hour in each system.
An example of genetic selection for leanness which caused handling problems in a slaughter plant was observed by both authors. Three hundred highly excitable fine-boned heifers of European Continental and British genetics, which had been selected for rapid growth and lean meat were observed. These heifers were impossible to handle calmly, and they sometimes engaged in self-destructive behavior. One heifer tore off her front leg and two others had ripped off pieces of a hoof. This occurred when they stepped in a crack between the loading ramp and the truck, which caused them to panic. Cattle with a calmer temperament will stop and withdraw their foot from the crack. We observed another heifer, which fell down and became so panicked that she thrashed around on the floor and was unable to make coordinated movements to get up. Another heifer pounded her head against the side of the chute after she fell down. The heifers constantly bellowed and if they were touched they kicked with both back feet. When a plant employee leaned over the side of the race to encourage a heifer to move, she responded by kicking with both back feet to a height of about 7 feet, which narrowly missed his head. One hind foot caught the brim of his hard hat with such force that it flew high in the air. These agitated behaviors have a strong genetic basis. Cattle with a calmer temperament do not display such frenzied, agitated behavior, even when they originate from a remote ranch and have had minimal previous contact with people. This type of behavior is similar to that of a high-strung horse. Horses are more likely to injure themselves when they panic compared to cattle. The first author has observed thousands of cattle move through this particular facility. In several years, she had never observed such berserk behavior.
The authors speculate that the increased problems with highly reactive and excitable cattle and pigs are the result of over selection for leanness and rapid growth. Over the last 20 years, the first author has observed a vast improvement in handling methods and equipment design for livestock in both the United States and Canada. This should theoretically lower the frequency of over reactive responses of livestock animals in novel situations. Unfortunately, this is not always the case. Indiscriminant selection for rapid growth and leanness may be related to increased handling problems.
Both authors hypothesize that there appears to be a relationship between body shape, bone structure, hair patterns, hair color, and temperament in cattle, pigs, and horses. During her career, the first author has handled and observed thousands of cattle and pigs in over 100 slaughter plants throughout the United States, Canada, and Europe. The second author has ridden, trained, and shod numerous horses. Both authors have observed that animals with a highly reactive temperament often have a lean, slender body and a fine-boned skeleton. The first author has observed that selecting pigs and cattle for lean meat and thin back fat has resulted in animals, which are more reactive to sudden novelty. Both authors have observed that it is the slender, smooth-bodied animals, which are most reactive. If animals are selected for leanness and bulging muscles they tend to be less reactive than the lean, smooth-bodied slender animals. However, both types of lean animals are probably more reactive than animals with more body fat. In Angus cattle, double-muscled animals had more excitable temperaments scores than normal animals (Holmes et al., 1972). The first author has observed that Charolais cattle bred in Quebec, Canada, with heavy bones and muscular bodies, are very calm at a noisy livestock auction compared to slender, lean Charolais cattle which are raised by some U.S. breeders. When we looked at the different body shapes and sizes of different breeds of pigs, cattle, and horses, the physique-temperament relationship became obvious. Arabian horses are lean, reactive, fine-boned, and spirited. Draft horses are muscular and heavy boned with mostly calm temperaments. When the draft horse breeds were developed it was likely that any horse which spooked and turned over the beer wagon was quickly eliminated. Early breeders probably selected for strong calm animals with heavy bones. Another example of the physique-temperament relationship is highly prolific Chinese pigs that have an abundance of fat and bear many offspring. These animals are very placid and nonreactive, whereas pigs, which carry the porcine stress gene slender, have low amounts of fat, and are more reactive. Pictrain pigs and the Chinese breeds are extreme examples of the relationship between temperament and body morphology. Temperament differences became obvious to both authors after observing hundreds of animals under different conditions.
Both authors further hypothesize that the size and shape of wild herd animals is also related to temperament. Highly reactive, delicate, lightweight antelope and deer survive in the wild by fleeing quickly from predators, and heavy animals like cattle and bison survive by either attacking or fleeing. However, the heaviest herding animal, the Cape buffalo, survives by attacking because it is too heavy to flee. The small, delicate, fine-boned, defenseless animals such as antelope have the most excitable temperaments, especially if they live in an environment with many predators. These animals are hyper vigilant and equipped with huge ears, which enable them to orient and localize faint sounds of danger. Any change in their environment will result in instant orientation and possible flight. The larger, heavier animals are more placid but they can be dangerous to handle in a confined space. If the handlers enter the animal's flight zone they are more likely to attack. The American bison is one of the most dangerous animals when it is cornered. The first author has observed bison butt a fence with their heads up to 20 times. They have also been known to eviscerate a horse with their upward curving horns. Bison appear to be intermediate in the survival strategy. They are very excitable and survive by fleeing, but since they are fairly heavy animals they often attack when they are unable to flee. Measurements of the stress hormone cortisol in a variety of hoofed animals has indicated that the large, heavy Cape buffalo is less stressed by capture and restraint than a flighty animal such as the nyala. When 18 different species of African animals were captured, the Cape buffalo was the only species in which cortisol levels did not rise (Morton et al., 1995).
Both authors have heard reports from dairymen that white holsteins are flighty and often more dangerous to handle than more pigmented cows. These casual observations made by dairymen may be explained partially by findings from studies on coat color-temperament relationships in other animals. Genetic selection for body traits has an effect on behavioral traits, and selection for behavioral traits leads to changes in body traits (Belyaev, 1979).
This phenomenon has clearly been demonstrated in rats and foxes (Keeler, 1942; Keeler et al., 1968). Breeding for the black non-agouti coat color in rats resulted in tamer, easier to handle rats (Keeler, 1942). In foxes, graded differences in temperament were found between foxes selected for different coat colors (Keeler, 1968, 1975). Foxes that were all raised in the same wooded compound had flight zone differences that ranged from silvers (200 yards), to platinums (100 to 200 yards), to ambers (100 to 3. yards). It is interesting to note that the foxes became progressively tamer as more and more mutant coat color genes were added. Tameness was also highly correlated with adrenal gland weights. The size of the adrenal decreases and the body weight increases as the foxes became tamer. The animals with the most mutant coat color genes were the tamest. Therefore, variations in coat color and hair patterns are related to behavior.
Many scientists believed that these observations were rubbish and that the trainers were wrong. Cattle have similar hair whorl patterns on their foreheads (Fig. 4.3). We decided to study hair whorls and temperament in cattle because the behavior of cattle is not confounded by the experience of being trained for riding. A total of 1500 cattle were temperament-ranked (with the rating scale that was described previously) while they were restrained in a hydraulic squeeze chute for vaccinations. The survey was conducted at a commercial feedlot in Colorado. Seventy-two percent of the cattle were European X British crosses and the rest were zebu crosses from Mexico. The animals originated from many different ranches. To help prevent bias the person determining the temperament rankings was positioned so that he could not see the hair whorl. A second observer noted hair whorl location.

Figure 4.3: The position of the round hair whorl on the animal's forehead is linked to temperament. Cattle with round hair whorls above the top of the eyes were more likely to become fearful and agitated when they were restrained.
The results of our study clearly showed that cattle with hair whorls above the eyes became significantly more agitated during restraint than cattle with hair whorls below the eyes (Grandin et aL, 1995). Further study of feedlot cattle, which had come from the same ranch, also indicated that animals with hair whorls above the eyes had larger flight zones and were more likely to entirely avoid a person who approached them.
The authors speculate that there may be a relationship between hair whorl position on the forehead and bone structure. Hair whorl height may possibly be related to increased vigilance, more acute senses, and a stronger orienting response. A low hair whorl may be related to reduced vigilance and a reduced orienting response. Bone structure may be related to overall nervous system reactivity and fearfulness. We speculate that these two aspects of temperament may have different neurotransmitter mechanisms. Possibly, the temperament of an animal is determined by more than one biological mechanism, which is influenced by different sets of genetic factors. In other words, orienting responses and a full-blown fear response may be influenced by different genetic mechanisms.
In most cattle and horses, the fine-boned, slender-bodied animals that resemble deer have a nervous temperament and a high hair whorl. The heavy-boned, muscular animals are more likely to have a calm temperament and a low hair whorl. The Holstein dairy cow lacks heavy muscling but fits the above criteria because she is heavy boned. We started to speculate about the possibility of two separate genetic mechanisms influencing temperament after the first author visited the Lasater Beefmaster herd. This herd has been closed to new genetics for 60 years and the cattle have been subjected to a unique set of selection pressures. Lasater (1972) selected his cattle using the natural principle of survival of the fittest. Heifers that were unable to give birth unassisted and protect their calf from the coyotes were culled. However, Lasater required cattle that were commercially useful so he also selected for temperament and carcass traits. The Beefmaster's breed is half Brahman, quarter Hereford, and quarter Shorthorn. Lasater's selection criteria resulted in cows, which are very protective of their calves but are extremely tame and seek contact with people. It is unusual to observe range cows that will come up and lick people and will stand still while being scratched and petted. The appearance of these reddish brown animals is striking. They are muscular and heavy boned and have either high hair whorls on the forehead or no hair whorls.
Dale Lasater, Tom Lasater's son, explained to these authors how they selected for temperament. Instead of using sudden aversive novelty (such as restraint) as a temperament test, they tested the temperament of hungry, newly weaned calves by sitting in the pen with them. Hungry calves, which failed to eat from a person's hand after 2 days, were culled. When the herd was first started about a quarter of the calves were culled; today, only 1% is culled for temperament (Dale Lassater, personal communication, 1996).
Faure and Mills (Chapter 8) demonstrated that the traits of fearfulness and social reinstatement are genetically separate. Social reinstatement is the tendency of an isolated bird to rejoin flockmates. In a wild population it is likely that both high fear and high social reinstatement naturally would appear together in the same animal because this would improve survival. In domestic cattle the high-strung, nervous animals will bunch together more tightly when they become excited. The tight bunching behavior is probably motivated by high fear. Social reinstatement is a trait that makes animals bond and that is probably not motivated by fear. Faure and Mills' social reinstatement trait is not the same as fear because they were able to select and breed both low-fear high-social reinstatement birds and vice versa. Another speculation is that Lasater duplicated the Faure and Mills' experiment in their cattle. The Beefmaster cattle have high bonding and good maternal traits but still have enough nervous system reactivity to be alert and protect their calves from predators. These high hair whorl, heavy-boned cattle are good mothers and there is a striking contrast between them and heavy-boned, low hair whorl Holstein dairy cows which are poor mothers. We further speculate that the high-fear may be more related to fine bone structure and the hair whorl position may be related to Faure and Mills' social reinstatement trait. As stated in earlier chapters, different traits within an animal are linked in ways that are not fully understood.
Lyons (1987) found that goats hand-reared by people react less strongly to changes in the environment compared to goats raised on their dams. Differ ences in early rearing methods resulted in long-term, stable differences in temperament. Several studies have shown that previous experience has a significant effect on an animal's flight zone size and its physiological responses to handling and restraint (Ewbank, 1993; Gonyou, 1993; Boissy and Bouissou, 1988; Ried and Mills, 1962; Hastings et al., 1992). For example, both hand-reared cattle and hand-reared deer show lower cortisol levels during restraint compared to animals with less early contact with people (Hastings et al., 1992; Boandle a al., 1989). Hastings et al. (1992) also found that "tame" hand-reared deer had much lower cortisol stress hormone levels when restrained compared to deer raised by their mothers. Interestingly, both groups actively resisted restraint and vocalized. In summary, Grandin (1984, 1987) suggests that compared to cattle having previous experience with rough handling, animals with previous experience with gentle handling will be calmer and easier to handle in the future.
In contrast to the highly reactive nyala or other reactive herd species, training placid animals such as Hereford cattle or Suffolk sheep to accept novel procedures can be done quickly and habituation to nonpainful procedures can occur even when the animals are pushed beyond the orienting response. However, if a highly reactive animal is trained too quickly and panics during the training, it may be very slow to habituate afterward. Highly reactive animals may become more fearful with each forced handling trial, whereas an animal with a more placid temperament may habituate and become less fearful. Grandin et al. (1994) found this in Angus X Hereford X Charlais X Simmental crossbred cattle, which became increasingly agitated when they were repeatedly run through a squeeze chute on the same day. Animals need time to calm down between training trials and practical experience has shown that training trials should be spaced at least 24 hours apart.
During handling and driving in alleys, lean hybrid pigs have been observed to startle frequently and are more easily excited compared to fat crossbred pigs (Grandin, 1994). The hybrids are highly reactive when a whip is cracked and raise their ears instantaneously in response to novel sounds. Lanier et al. (1995) conducted experiments on genetically similar crossbred pigs with identical rearing experiences and found individual differences in their adaptability to a forced novel swimming task. Some of the pigs readily adapted to a series of swimming tasks and others remained highly stressed. Some of the pigs had baseline epinephrine (adrenalin) levels after swimming, and others still had high levels. In essence, the stressed pigs never adapted. To summarize, animals with a placid, calm temperament will habituate more easily to forced, nonpainful procedures than animals with a flighty, excitable temperament.
To facilitate handling and adaptability to commercial conditions, the first author has experience training market-weight pigs with excitable genetics to drive quietly at the slaughter plant. The training is done on the farm during the entire finishing time (fattening period). She found that walking through the pens in a random pattern each day and training the pigs to move away in an orderly manner, only 10 to 15 seconds per pen was needed each day. The pigs learned to get up and flow around her as she walked through the pen. In this manner, they are taught to be driven quietly by people instead of learning to follow them. However, Grandin suggests that care must be taken by the person walking the pens not to frighten the pigs. The idea is to teach the pigs to become accustomed to people and to get up and move when people enter the pen, but not to get so upset that they panic. Walking the pens is especially beneficial for lean hybrids with nervous temperaments. Cattle-handling specialist Bud Williams from Canada and Burt Smith from Hawaii (T. Grandin, personal communication) have used similar training methods with wild and domestic ruminants on pastures. These experiences suggest that herding animals can be taught to either follow people or allow themselves to be driven. In some farming systems, following is more efficient, while in others driving is preferable.
Emotionally reactive animals are often in a high state of nervous system arousal due to the novelty of the situation, which makes them more vigilant and likely to balk at small distractions in alleys and races that draw their attention. A jiggling chain, people up ahead, a sparkling reflection on a piece of metal, or air drafts and hissing exhausts blowing in their faces can all cause the animals to stop (Grandin, 1993). More placid animals will usually hesitate and look at small distractions, but they are more likely to keep moving. However, an area of high contrast or something that looks out of place is more likely to cause balking in nervous animals. In five different slaughter plants, the removal of distractions such as sparkling reflections, air drafts, and the presence of people up ahead made it possible to greatly reduce the use of electric prods (Grandin, 1996).
The managers of bull test stations have reported two accounts of bulls that went berserk when brought to the station. The bulls charged fences and attacked the handlers, but afterward, the owner was able to walk them quietly into a trailer. They calmed down when they heard the owner's familiar voice. In horses, both authors observed that the true temperament can be masked by learning. Some horses "blow up" when they are taken to a new place or exposed to a strange stimulus. For example, owners often teach their young horses to allow their feet to be handled by a horseshoer. The horse may learn to stand and accept this handling by the owner but when the farrier arrives for the first time the horse may panic. Some horses become fearful every time they are handled by a farrier. Owners often comment that this is "out of character" for the horse. When highly reactive animals are in familiar surroundings they may be calm and their true temperament can be masked. They learn that that certain sights and sounds are harmless. Recently, the owner of a high-strung horse commented, "He's 12 years old. You'd think he learn to get over the little things by now." Taming and training adult animals may reduce their reactions to specific handling procedures but it does not change their level of innate emotional reactivity The level of emotional reactivity in an animal is like the level of water in a drinking glass. The level of water in the glass is influenced by both genetics and the environment during early life. Environmental factors can either raise or lower the initial level of water in the glass. Handling and contact with people early in life can reduce emotionality in an adult animal but it is almost impossible to manipulate the environment to such an extent that a highly reactive animal can become as calm as an animal which has calm genetics. For example, no amount of early handling can turn a high-strung Arab stallion into an "old plug" riding stable horse. You might make him calmer, but he will never be as calm as horse that is genetically calm.
Herd animals respect a barrier that appears visually substantial (Grandin, 1993). Practical experience shows that the use of solid barriers is most important for highly fearful or reactive animals. People handling cattle, deer, sheep, and wild horses have independently discovered these principles (Grandin, 1980a; Kilgour, 1971; Fowler, 1978). Herding animals seek safety behind barriers. Maybe it is like seeking safety from predators in the middle of the herd. Whittington and Chamove in New Zealand (1995) found that installing solid barriers on a deer pasture reduced vigilance. Solid barriers also reduced the tendency of deer to react by running away from a moving novel stimulus. The novel stimulus used in this study was a person draped with a sheet.
Tulloh (1961) showed that Brahman cattle are more difficult to block with gates compared to British breeds. When Brahman cattle are running in an alleyway they do not see the fence. Adding a 30-cm metal strip or tying ribbons to a fence can prevent the animals from hitting it. When either wild or domestic herd animals panic, they are likely to crash into fences constructed of thin rods or chain link which are difficult to see.
Grandin (1993) found that extensively raised cattle remain quieter and become less agitated (fearful) when the sides of restraint devices are covered with solid material such as metal, plywood, or cardboard. Solid barriers on the side of a squeeze chute prevent the animal from seeing the chute operator who stands deqi in the animal's flight zone (Fig. 4.4). Squeeze chute operators have to stand next to the machine with their arms held high over their heads to put their hands on the controls. Seeing people in this position often makes cattle lunge and jump in attempt to escape (Fig. 4.5). Installing cardboard on the outside of the squeeze chute can substantially reduce agitated behavior and less pressure will be required to hold the animal. All species will stand more quietly in a restraint device if pressure is applied with a slow steady motion. Sudden jerky motions of a restraint device cause excitement. There is also an optimal pressure. The restraint device must apply sufficient pressure to provide the feeling of restraint, but excessive pressure which would cause pain should be avoided.

Figure 4.4 Rubber louvers on the side of the squeeze chute prevent the cattle from seeing people deep in their flight zone. The sides are designed so that the handler can access the animal for vaccinations, but its vision is blocked.
Parsons and Helphinstine (1969) used a dark box for handling cattle, and Pollard and Littlejohn (1994) and Pollard et al. (1992) used a dark room for deer. Deer in a dark room lose their flight zone and can be touched by people. When a handler was present in the dark room, the deer bunched together less tightly and investigated more compared to deer in a lighted room. The first author has observed dramatic effects of darkness on bleshock antelope. Placing the antelope in a completely dark crate stopped it from kicking. If it saw a small amount of light coming through the air holes, it kicked violently In order to calm frightened horses, the second author has had success with covering their eyes with a soft blanket. This has to be approached slowly, but when the horse accepts having its eyes covered, the calming effect is obvious.
Cattle and other hoofed animals will often refuse to enter a race or restraint device if it looks like a dead end. Cattle entering a restraint device must see a lighted place to put their heads, but if they see an avenue of escape they are more likely to become agitated. The first author has observed that extensively raised cattle will remain quieter in a restraint device if there is also a solid barrier around the animal's head. This is especially important with flighty animals which are not accustomed to close contact with people.
Amaral, A. (1977). "Mustang Life and Legends of Nevada's Wild Horses." University of Nevada Press, Reno.
Arave, C. W, Albright, J. L., and Sinclair, C. L. (1974). Behavior, milk yield and leucocytes of dairy cows in reduced space and isolation. J. Dairy Sci. 59, 1497-1501.
Barker, R. (1990). The mind behind the swirls. Rocky Mt. Quarter Horse 28, March, 26-27.
Beatie, V E., Walker, N., and Sneddon, I. A. (1995). Effect of rearing environment and change of environment on the behavior of gilts. Appl. Anim. Behav. Sci. 46, 57-65.
Belyaev, D. K. (1979). Destabilizing selection as a factor in domestication. J. Hered. 70, 301-308.
Boandle, K. B., Wohlt, J. E., and Carsia, R. V (1989). Effect of handling, administration of local anesthetic and electrical de-horning on plasma cortisol in Holstein calves. J. Dairy Sci. 72, 2193-2197.
Boissy, A. (1995). Fear and fearfulness in animals. Q. Rev. Bid. 70, 165-191.
Boissy, A., and Bouissou, M. (1988). Effects of early handling on heifers' subsequent reactivity to humans and to unfamiliar situations. Appl. Anim. Behav. Sci. 20, 259-273.
Boissy, A., and Le Neindre, P. (1990). Social influences on the reactivity of heifers: Implications for learning abilities in operant conditioning. Appl. Anim. Behav. Sci. 25, 149-165.
Boissy A., Le Neindre, P., Orgeur, P., and Bouix, J. (1996). Genetic variability psychobiological reactivity of lambs reared under open range management Proc. mt. Congr mt. Soc. Appl. Ethol., 30th; University of Guelph, Guelph, Ontario, Canada, 1996, p. 59.
Boivin, X., Le Neindre, P., Chupin, J. M., Garel, J. P., and Trillat, G. (1992). Influence of breed and early management on ease of handling and open-field behavior of cattle. Appl. Anim. Behav. Sci. 32, 313-323.
Bonsma, J. (1975a). Judging cattle for functional efficiency Beef Cattle Sci. Handb. 12, 23-36, papers delivered at the International Stockmen's School, San Antonio, TX.
Bonsma, J. (1975b). Crossbreeding for increased cattle production. Beef Cattle Sci. Handb. 12, 37-49; papers delivered at the International Stockmen's S4iool, San Antonio, TX.
Broadhurst, P. L. (1960). Analysis of maternal effects in the inheritance of behavior. Anim. Behav. 9, 129-141.
Coppinger, L., and Coppinger, R. (1993). Dogs for herding livestock. In "Livestock Handling and Transport" (T. Grandin, ed.), pp. 179-196. CAB International, Wallingford, UK.
Dantzer, R., and Mormede, P (1978). Behavioral and pituitary-adrenal characteristics of pigs differing in their susceptibility to the malignant hypothermia syndrome induced by halothane anesthesia. Ann. Rech. Vet. 9(3), 559-567.
Davis, M. (1992). The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 15, 353-375.
DeFries, J. C., Gervais, J. C., and Thomas, B. A. (1978). Response to 30 generations of selection for open field activity in laboratory mice. Behav. Genet. 8(1), 3-13.
Dellmeier, G. R., Friend, T. H., and Gbur, E. B. (1985). Comparison of four methods of calf confinement. II. Behavior J. Anim. Sci. 60, 1102-1109.
Denenberg, V H., and Whimbey, A. E. (1968). Experimental programming of life histories: Towards an experimental science of individual differences. Devel. Psychobiol. 1(1), 55-59.
Denenberg, V H., Brumaghim, J. T., Haltmeyer, G. C., and Zarrow, M. X. (1967). Increased adrenocortical activity in the neonatal rat following handling. Endocrinology, (Baltimore) 81, 1047-1052.
Dickson, D. P, Barr, G. R.,Johnson, L. P, and Wiekert, D. A. (1970). Social dominance and temperament in dairy cows. J. Dairy Sci. 53, 904.
Douglass, A. G., Darre, M. D., and Kinsman, D. M. (1984). Sight restriction as a means reducing stress during slaughter. Proc. Euro. Meet. Meat Res. Workers, 30th, Bristol, UK, pp. 10-11.
Ewbank, R. (1993). Handling cattle in intensive systems. In "Livestock Handling and Transport" (T. Grandin, ed.), pp. 59-73. CAB International, Wallingford, UK.
Eysenck, H. J., and Broadhurst, P L. (1964). Experiments with animals: Introduction. In "Experiments in Motivation" (H. J. Eysenck, ed.), pp. 285-291. Macmillan, New York.
Fordyce, G. (1987). Weaner training. Queensl. Agric. J. 113, 323-324.
Fordyce, G., Goddard, M. B., Tyler, R., Williams, G., and Toleman, M. A. (1985). Temperament and bruising of Bos indicus cross cattle. Aust. J. Exp. Agric. 25, 283-288.
Fordyce, G., Dodt, R. M., and Wyther, J. R. (1988). Cattle temperaments in extensive herds in northern Queensland. Aust. J. Exp. Agric. 28, 683-687.
Fowler, M. B. (1978). "Restraint and Handling of Wild and Domestic Animals:' Iowa University Press, Ames.
Friedly, J. (1990). Dang... there's a tricoglyph on your horse. Rocky Mt. Quarter Horse 28(July), 26-27.
Fuller,J. L., and Thompson, W R. (1978). "Foundations of Behavior Genetics." Mosby, St. Louis, MO.
Gonyou, H. W (1993). Behavioral principles of animal handling and transport. In "Livestock Handling and Transport" (T. Grandin, ed.). CAB International, Wallingford, UK.
Grandin, T. (1980a). Livestock behavior related to handling facilities design. Int. J. Study Anim. Prob. 1, 33-52.
Grandin, T. (1980b). Observations of cattle behavior applied to the design of handling facilities. Appl. Anim. Ethol. 6, 19-33.
Grandin, T. (1984). Reduce stress of handling to improve productivity of livestock. Vet. Med. 79, 287-831.
Grandin, T. (1987). Animal handling. Vet. Clin. North Am. 3, 323-338.
Grandin, T. (1989). Effects of rearing environment and environmental enrichment on behavior and development of young pigs. Doctoral Dissertation, University of Illinois, Urbana-Champaign.
Grandin, T. (1992). Behavioral agitation is persistent over time. Appl. Anim. Behav. Sci. 36, 1-9.
Grandin, T. (1993). Behavioral principles of cattle handling under extensive conditions. In "Livestock Handling and Transport" (T. Grandin, ed.), pp. 43-57. CAB International, Wallingford, UK.
Grandin, T. (1994). Solving livestock handling problems. Vet. Med. 89, 989-998.
Grandin, T. (1996). Factors which impede animal movement in slaughter plants. J. Am. Vet. Med. Assoc. 209, 757-759.
Grandin, T. (1997). Assessment of stress during handling and transport. J. Anim. Sci. 75, 249-257.
Grandin, T., Curtis, S. E., Widowski, T. M., and Thurman, J. C. (1986). Electro-immobilization versus mechanical restraint in an avoidance choice test. J. Anim. Sci. 62, 1469-1480.
Grandin, T., Curtis, S. E., and Taylor, I. A. (1987). Toys, mingling and driving reduce excitability in pigs. J. Anim. Sci. 65, Suppl. 1, 230 (abstr.).
Grandin, T., Odde, K. G., Schutz, D. N., and Behrns, L. M. (1994). The reluctance of cattle to change a learned choice may confound preference tests. Appl. Anim. Behav. Sci. 39, 2 1-28.
Grandin, T., Rooney, M. B., Phillips, M., Cambre, R. C., Irlbeck, N. A., and Graffam, W (1995). Conditioning of nyala (Tragelaphus angasi) to blood sampling in a crate with positive reinforcement. Zoo Biol. 14, 261-273.
Grandin, T., Deesing, M. J., Struthers, J. J., and Swinker, A. M. (1995). Cattle with hair whorl patterns above the eyes are more behaviorally agitated during restraint. Appl. Anim. Behav. Sci. 46, 117-123.
Grandin, T., Irlbeck, N., and Phillips, M. (1996). Training antelope to cooperate with veterinary procedures. Proc. Int. Congr. Int. Soc. Appl. Anim. Ethol., 30th, University of Guelph, Guelph, Ontario, Canada.
Hale, R. L., Friend, T. H., and Macauley, A. S. (1987). Effect of method of restraint on cattle on heart rate, cortisol and thyroid hormone. J. Anim. Sci. (Suppl. 1) (Abstract) 219-220.
Harger, C. M. (1928). "Frontier Days." Macrae Smith Company, Philadelphia.
Hastings, B. E., Abbot, D. E., George, L. M., and Stradler, S. G. (1992). Stress factors influencing plasma cortisol levels and adrenal weights in Chinese Water deer. Res. Vet. Sci. 53, 375-380.
Hedigar, H. (1955). "Studies of the Psychology and Behavior of Captive Animals in Zoos and Circuses." Criterion Books, New York.
Hedigar, H. (1968). "The Psychology and Behavior of Animals in Zoos and Circuses." Dover, New York.
Hemsworth, P. H., Barnett, J. L., Hansen, C., and Gonyou, H. W (1986). The influence of early contact with humans on subsequent behavioral response of pigs to humans. Appl. Anim. Behav. Sci. 15, 55-63.
Hemsworth, P. H., Barnett, J. L., Treasy, D., and Madgwick, P (1990). The inheritability of the trait fear of humans and the association between this trait and subsequent reproductive performance of gilts. Appl. Anim. Behav. Sci. 25, 85-95.
Hirsh, J. (1963). Behavior genetics and individuality understood. Science 142, 1436-1442.
Hohenboken, W D. (1987). Behavioral genetics. Vet. Clin. North Am. Food Anim. Prac. 3, 217-229.
Holmes, J. H. G., Robinson, D. W, and Ashmore, C. R. (1972). Blood lactic acid and behavior in cattle with hereditary muscular hypertrophy J. Anim. Sci. 35, 1011-1013.
Huck, U. W, and Price, E. 0. (1975). Differential effects of environmental enrichment on the open-field behavior of wild and domestic Norway rats. J. Comp. Physiol. Psychol. 89, 892-898.
Hutson, G. D. (1980). Visual field, restricted vision and sheep movement in laneways. Appl. Anim. Ethol. 6, 233-240.
Hutson, G. D. (1993). Behavioral principles of sheep handling. In "Livestock Handling and Transport" (T. Grandin, ed.), pp. 127-146. CAB International, Wallingford, UK.
Keeler, C. E. (1942). The association of the black (non-agouti) gene with behavior in the Norway rat. J. Hered. 33, 371-384.
Keeler, C. E. (1975). Genetics of behavior variations in color phases of the red fox. In "The Wild Canids: Their Systematics, Behavioral Ecology and Evolution" (M. W. Fox, ed.), p. 1. Van Nostrand-Reinhold, New York.
Keeler, C. E. (1968). Coat color, physique, and temperament. J. Hered. 59, 82-84.
Keeler, C. E., Ridgway, S., Lipscomb, L., and Fromme, E. (1968). The genetics of adrenal size and tameness in color phase foxes. J. Hered. 59, 82-84.
Kerr, S. G. C., and Wood-Gush, D. G. M. (1985). Investigation of relationships between behavioral and production measures of dairy heifers. Appl. Anim. Behav. Sci. 15, 181-182.
Kerr, S. G. C., and Wood-Gush, D. G. M. (1987). The development of behavior patterns and temperament in dairy heifers. Behav. Processes 15, 1-16.
Kiley-Worthington, M. (1976). Tail movements of ungulates, canids and felids with particular reference to their causation and function as displays. Behaviour 56, 69-115.
Kilgour, R. (1971). Animal handling in works: Pertinent behavior studies. Proc. Meat Ind. Res. Conf. 13th, Hamilton, NZ, pp. 9-12.
Kilgour, R. (1975). The open-field test as an assessment of the temperament of dairy cows. Anim. Behav. 23, 615-624.
Kilgour, R., and Dalton, D. C. (1984). "Livestock Behaviour." University of New South Wales Press, Sydney, Australia.
Kilgour, R., and de Langen, H. (1970). Stress in sheep resulting from management practices. Proc. N. Z. Soc. Anim. Prod. 30, 65-76.
Krushinski, L. V (1960). In "Animal Behavior. Its Normal and Abnormal Development" (J. Wortis, ed.). International Behavioural Sciences Service, Consultants Bureau, New York. (Original Russian version published by Moscow University Press).
Lasater, L. M. (1972). "The Lasater Philosophy of Cattle Raising." University of Texas Press, El Paso.
Lanier, E. K., Friend, T. H., Bushong, D. M., Knabe, D. A., Champney, T. H., and Lay D. G. (1995). Swine habituation as a model for eustress and distress in the pig. J. Anim. Sci. 73, Suppl. 1, 126 (abstr.).
Lawrence, A. B., Terlouw, E. M. C., and Illius, A. W (1991). Individual differences in behavioral responses of pigs exposed to non-social and social challenges. Appl. Anim. Behav. Sci. 30, 73-78.
Lay, D. C., Friend, T. H., Grissom, K. K., Hale, R. L., and Bowers, C. C. (1992). Novel breeding box has variable effect on heartrate and cortisol response of cattle. J. Anim. Sci. 35, 1-10.
Le Neindre, P., Trillat, G., Sapa, F, Menissier, F, Bonnet,J. N., and Chupin,J. M. (1995). Individual differences in docility of Limousin cattle. J. Anim. Sci. 72, 2249-2253.
Levine, L., Grossfield, J., and Rockwell, R. F (1979). Functional relationships between genotypes and environment in behavior. J. Hered. 70, 317-320.
Levine, S. (1968). Influence of infantile stimulation on the response to stress during preweaning development. Dev. Psychobiol. 1(1), 67-70.
Levine, S., Haltmeyer, G. C., Karas, G. G., and Denenburg, V H. (1967). Physiological and behavioral effects of infantile stimulation. Phsyiol. Behav. 2, 55-59.
Lynch, J. J., and Alexander, G. (1973). "The Pastoral Industries of Australia." University Press, Sydney, Australia.
Lyons, D. M. (1987) Individual differences in temperament of dairy goats and the inhibition of milk ejection. Appl. Anim. Behav. Sci. 22, 269-282.
Mal, M. E., and McCall, C. A. (1996). The influence of handling during different ages on a halter training test in foals. Appl. Anim. Behav. Sci. 50, 115-120.
Matthews, L. R. (1993). Deer handling and transport. In "Livestock Handling and Transport" (T. Grandin, ed.), pp. 253-272. CAB International, Wallingford, UK.
Mayr, E. (1958). Behavior and systematics. In Behavior and Evolution (A. Roe and G. G. Simpson, eds.), pp. 341-362. Yale University Press, New Haven.
McCann, J. S., Heird, J. C., Bell, R. W, and Lutherer, L. 0. (1988a). Normal and more highly reactive horses. I. Heart rate, respiration rate and behavioral observations. Appl. Anim. Behav. Sci.19, 201-214.
McCann, J. S., Bell, R. W, and Lutherer, L. 0. (1988b). Normal and more highly reactive horses. II. The effects of handling and reserpine on the cardiac response to stimuli. Appl. Anim. Behav. Sci. 19, 215-226.
Melzak, R. (1954). The genesis of emotional behavior. An experimental study of the dog. J. Comp. Physiol. Psychol. 47, 156-168.
Melzak, R., and Burns, S. K. (1965). Neurophysiological effects of early sensory restriction. Exper. Neurol. 13, 163-175.
Morton, D. J., Anderson, E., Foggin, C. M., Kock, M. D., and Tiran, E. P. (1995). Plasma cortisol as an indicator of stress due to capture and translocation in wildlife species. Vet. Rec. 136, 60-63.
Murphey, R. M., Moura Duarte, F A., and Torres Penedo, M. C. (1980). Approachability of bovine cattle in pastures: Breed comparisons and a breed X treatment analysis. Behav. Genet. 10, 171-181.
Murphey, R. M., Moura Duarte, F A., and Torres Penedo, M. C. (1981). Responses of cattle to humans in open spaces: Breed comparisons and approach-avoidance relationships. Behav. Genet.11, 37-48.
O'Blesness, G. V, Van Vleck, L. D., and Henderson, C. R. (1960). Heritabilities of some type appraisal traits and their genetic and phenotypic correlations with production. J. Dairy Sci. 42,1490-1498.
Orihuela, J. A., and Solano, J. J. (1994). Relationship between order of entry in slaughterhouse raceway and time to traverse raceway Appl. Anim. Behav. Sci. 40, 3 13-317.
Parsons, P. A. (1988). Behavior, stress, and variability Behav. Genet. 18, 293-308.
Parsons, R. A., and Helphinstine, W N. (1969). "Rambo A. I. Breeding Chute for Beef Cattle," One Sheet Answers. University of California Agricultural Extension Service, Davis.
Pascoe, P J. (1986). Humaneness of an electroimmobilization unit for cattle. Am. J. Vet. Res. 10, 2252-2256.
Pederson, B. K. (1992). Comprehensive evaluation of well being in pigs: Environmental enrichment and pen space allowance. Ph.D. Thesis, University of Illinois, Urbana.
Plomin, R. (1990). The role of inheritance in behavior. Science 248, 183-88.
Pollard, J. C., and Littlejohn, R. P. (1994). Behavioral effects of light conditions on red deer in holding pens. Appl Anim. Behav. Sci. 40, 127-34.
Pollard,J. C., Littlejohn, R. P.,Johnstone, P, Lass, EJ., Carson, I. D., and Suttie,J. M. (1992). Behavioral and heart rate response to velvet antler removal in red deer. N. Z. Vet.J 40, 56-61.
Price. E. 0. (1984). Behavioral aspects of animal domestication. Q. Rev. Biol 59, 1-2.
Prince, J. H. (1977). The eye and vision. In "Duke's Physiology of Domestic Animals" (M. J. Swenson, ed.). Cornell University Press, Ithaca, NY.
Ried, R. L., and Mills, S. C. (1962). Studies of carbohydrate metabolism of sheep. XVI. The adrenal response to physiological stress. Aust.J Agric. Res. 13, 282-94.
Roche, B. W (1988). Coacher mustering. Queensl Agric.J 114, 215-16.
Rogan, M. T., and LeDoux, J. E. (1996). Emotion: Systems, cells, and synaptic plasticity. Cell (Cambridge, Mass.), 85, 369-475.
Sato, S. (1981). Factors associated with temperament in beef cattle. Jpn. J. Zootech. Sci. 52, 595-605.
Schupe, W L. (1978). "Transporting Sheep to Pastures and Market," Tech. Pap. No. 78-6008. Am. Soc. Agric. Eng., St. Joseph, MI.
Scott, J. P., and Fuller, J. L., (1965). "Genetics and the Social Behavior of the Dog." University of Chicago Press, Chicago.
Seabrook, M., ed. (1987). "The Role of the Stockman in Livestock Productivity and Management," Proc. Semin., Rep. EUR 10982 EN. Commission of the European Communities, Luxemburg.
Shrode, R. R., and Hammack, S. P (1971). Chute behavior of yearling beef cattle. J. Anim. Sci. 33, 193 (absr.).
Smith, D. W, and Gong, B. T. (1973). Scalp hair patterning as a clue to early fetal development. J. Pediatr 83, 374-380.
Smith, D. W, and Gong, B. T. (1974). Scalp hair patterning: Its origin and significance relative to early fetal brain development. Teratology 9, 17-34.
Stricklin, W R., Heisler, C. E., and Wilson, L. L. (1980). Heritability of temperament in beef cattle. J. Anim. Sci. 51, Suppl. 1, 109 (abstr.).
Syme, L. A., and Elphick, G. R. (1982). Heart-rate and the behavior of sheep in yards. Appl. Anim. Ethol. 9, 31-35.
Tanner, M., Grandin, T., Cattell, M., and Deesing, M. (1994). The relationship between facial hair whorls and milking parlor side preference. J. Anim. Sci. 72, Suppl. 1, 207 (abstr.).
Tellington-Jones, L., and Bruns, V. (1985). "The Tellington-Jones Equine Awareness Method." Breakthrough Publications, Millwood, NY.
Torres-Hernandez, G., and Hohenboken, W (1979). An attempt to assess traits of emotionality in crossbred ewes. Appl. Anim. Ethol. 5, 71-83.
Tulloh, N. M. (1961). Behavior of cattle in yards. II. A study of temperament. Anim. Behav. 9, 25-30.
Van Putten, G., and Elshof, W J. (1978). Observations on the effects of transport on the well being and lean quality of slaughter pigs. Anim. Regul. Stud. 1, 247-271.
Voisinet, B. D., Grandin, T., Tatum, J. D., O'Conner, S. F, and Struthers, J. (1997). Feedlot cattle with calm temperaments have higher average daily gains than cattle with excitable temperaments. J. Anim. Sci. 75, 892-896.
Wagnon, K. A., Loy, R. G., Rollins, W C., and Carroll, F D. (1966). Social dominance in a herd of Angus, Hereford and Shorthorn cows. Anim. Behav. 14, 474-479.
Whateley, J., Kilgore, R., and Dalton, D. C. (1974). Behavior of hill country sheep breeds during farming routines. Proc. N. Z. Soc. Anim. Prod. 34, 28-36.
Whittington, C. J., and Chamove, A. S. (1995). Effects of visual cover on farmed red deer behavior. Appl. Anim. Behav. Sci. 45, 309-314.
Whittlestone, W G., Kilgore, R., de Langren, H., and Duirs, G. (1970). Behavioral stress and the cell count of bovine milk. J. Milk Food Technol. 33, 217-220.
Willham, R. L., Karas, G. G., and Henderson, D. C. (1964). Partial acquisition and extinction of an avoidance response in two breeds of swine. J. Comp. Physiol. Psychol. 57, 117-122.
Wright, S. (1978). The relation of livestock breeding to theories of evolution. J. Anim. Sci. 46, 1192-1200.
Wyman, W D. (1946). "The Wild Horse of the West." Caxton Printers, Caldwell, ID.
Zavy, M. T., Juniewicz, P E., Williams, A. P., and von Tungeln, D. L. (1992). Effects of initial restraint, weaning and transport stress on baseline and ACTH stimulated cortisol responses in beef calves of different genotypes. Am. J. Vet. Res. 53, 552-557.
 Click here to return to the Homepage for more information on animal behavior, welfare, and care.
Click here to return to the Homepage for more information on animal behavior, welfare, and care.